Decentralized Clinical Trial –
1,400+ Participants
The Challenge
I was challenged with finding participants for a decentralized clinical study to evaluate an at-home colon cancer screening test. The client asked me to find a minimum of 1,000 participants for a multi-step trial had various inclusions and requirements.
Must be at least 45 years old
No colonoscopy in the last ten years
Average risk of colon cancer
Requirements
Collect then return an at-home stool sample via a provided kit
Schedule, attend and self-finance an in-office colonoscopy procedure with a gastroenterologist of their choice
Assist with the medical records collection process
Inclusion & Exclusion
Pre-study Pilot: I developed and conducted a smaller scale pilot to refine processes and help determine which approach was best suited
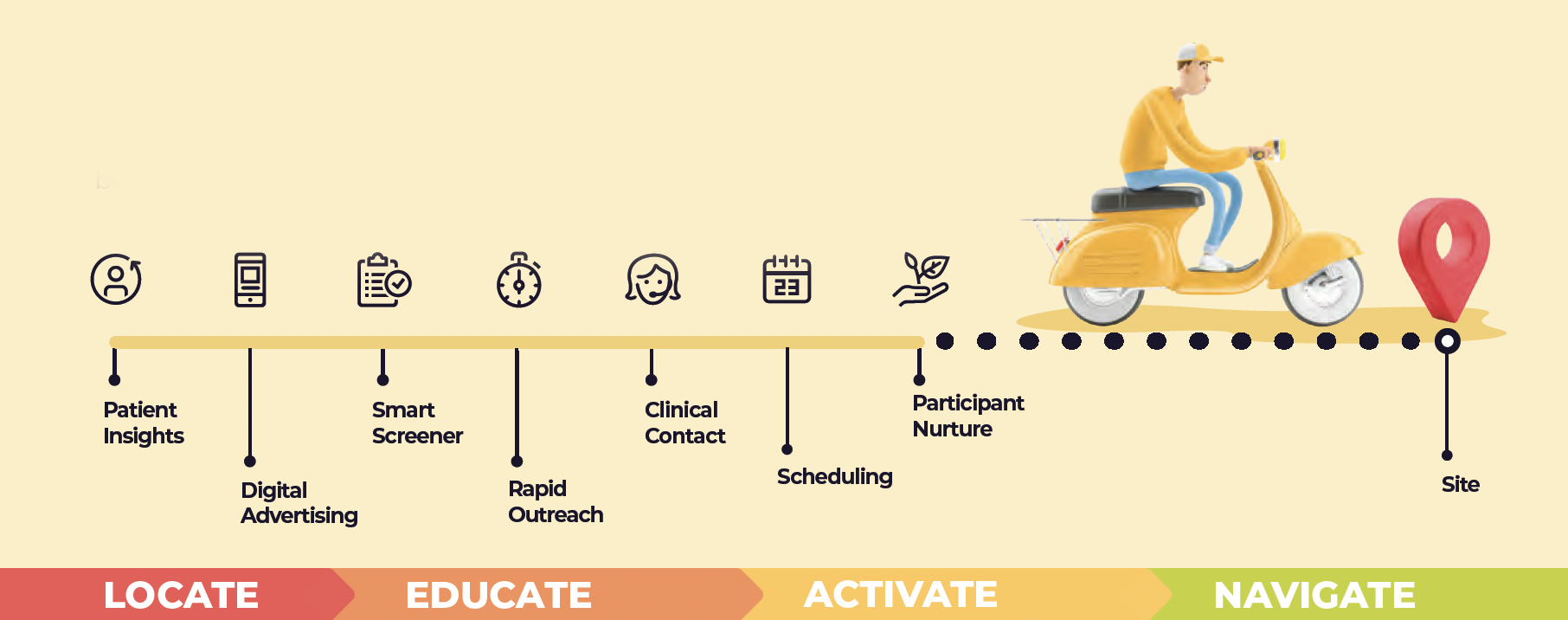
Project Strategy: I designed an end-to-end project for decentralized study recruitment using the LEAN process: Locate, Educate, Activate, Navigate
The Results
This successful project resulted in 1,402 trial completions, 40% more than requested. My client was also able to secure “Breakthrough Device” designation from the FDA and funding for an additional large pivotal study. Additional benefits included:
66% timeline reduction
1,400+ diverse participants set
1st participant final analysis in under 6 months
Dataset finalization and analysis completed in 5 days
The Strategy
With so many moving parts, it was essential that all participant activity be trackable in real time, then documented and time stamped.
The Process:
Client Satisfaction:
“We are so grateful that we chose this agency to assist with our clinical trials. They’ve dramatically improved our ability to locate and manage a high volume of qualified patients in a short window of time. This agency provided a streamlined, end-to-end solution to efficiently recruit patients in a highly efficient and cost-effective manner. These contributions will help us to deliver innovative technology to patients and physicians on an expedited pathway.”
Andrew Barnell | CEO